

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1
You know the right answer?
Pure water has a hydrogen ion concentration of 1 × 10−7 m and a ph of 7. a student measures a basic...
Questions




Mathematics, 29.11.2020 21:30

Computers and Technology, 29.11.2020 21:30

Mathematics, 29.11.2020 21:30

Biology, 29.11.2020 21:30


English, 29.11.2020 21:30

Mathematics, 29.11.2020 21:30

Mathematics, 29.11.2020 21:30


Computers and Technology, 29.11.2020 21:30




Mathematics, 29.11.2020 21:30



Mathematics, 29.11.2020 21:30

![[H^+]](/tpl/images/0294/9707/07acb.png) and
and ![[OH^-]](/tpl/images/0294/9707/b2910.png) .
.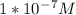 then hydroxide ion concentration must also be
then hydroxide ion concentration must also be 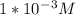 which is totally an error.
which is totally an error. ![pH=-log[H^+]](/tpl/images/0294/9707/15713.png)


