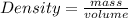Chemistry, 20.09.2020 05:01 tonehasarathi
An unknown solid has a mass of 837.21 g. The volume of the solid, determined by water displacement is 37.00 mL. Calculate the density of the solid.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
An unknown solid has a mass of 837.21 g. The volume of the solid, determined by water displacement i...
Questions




Social Studies, 29.07.2019 13:20

Geography, 29.07.2019 13:20



Mathematics, 29.07.2019 13:20

Biology, 29.07.2019 13:20


History, 29.07.2019 13:20


History, 29.07.2019 13:20



Computers and Technology, 29.07.2019 13:20


Mathematics, 29.07.2019 13:20

Mathematics, 29.07.2019 13:20

Health, 29.07.2019 13:20