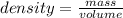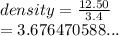Chemistry, 20.09.2020 02:01 3ittylilbiddy3
If a substance has a mass of 12.50 g and takes up 3.4 mL of space, what is the density?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
If a substance has a mass of 12.50 g and takes up 3.4 mL of space, what is the density?...
Questions



History, 28.01.2020 14:55



Social Studies, 28.01.2020 14:55

Chemistry, 28.01.2020 14:55


History, 28.01.2020 14:55


Mathematics, 28.01.2020 14:55



Mathematics, 28.01.2020 14:55




Biology, 28.01.2020 14:55

Mathematics, 28.01.2020 14:55

Mathematics, 28.01.2020 14:55