
Chemistry, 20.09.2020 02:01 zanestone12
The vapor pressure of carbon disulfide is 355.6 torr at 25°C. What is the vapor pressure of a solution prepared by dissolving 10.60 g naphthalene (C10H8, Molar Mass = 128.2 g/mol) in 155 mL CS2 liquid (Molar Mass = 76.14 g/mol, density = 1.261 g/mL)? Assume the solution obeys Raoult's law, and treat naphthalene as a nonvolatile solute.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
The vapor pressure of carbon disulfide is 355.6 torr at 25°C. What is the vapor pressure of a soluti...
Questions


Biology, 09.11.2020 17:30


Physics, 09.11.2020 17:30

Mathematics, 09.11.2020 17:30


Mathematics, 09.11.2020 17:30

Mathematics, 09.11.2020 17:30

Mathematics, 09.11.2020 17:30


Physics, 09.11.2020 17:30

History, 09.11.2020 17:30

Mathematics, 09.11.2020 17:30

Mathematics, 09.11.2020 17:30

Mathematics, 09.11.2020 17:30


English, 09.11.2020 17:30



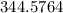 torr
torr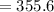 torr
torr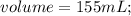
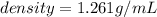
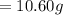
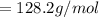
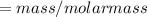
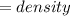 ×
× 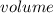
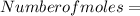
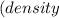 ×
× 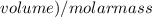
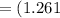 ×
× 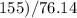
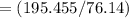
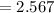 moles of Carbon Disulfide
moles of Carbon Disulfide
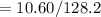
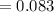
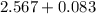
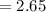 moles
moles
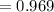
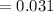
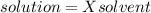 ×
× 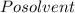
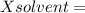 Mole fraction of solvent
Mole fraction of solvent
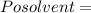 Vapour pressure of the pure solvent
Vapour pressure of the pure solvent
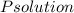
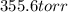
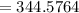 torr
torr

