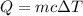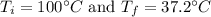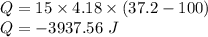Chemistry, 20.09.2020 05:01 armahoney8566
3. Burns from boiling water can be severe, caused by the transfer of energy from the boiling water to the
skin as the water cools to body temperature. How much heat (kJ) is transferred from the from 15.0g
of boiling water at 100.0°C as it hits the skin and cools to 37.2°C?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
You know the right answer?
3. Burns from boiling water can be severe, caused by the transfer of energy from the boiling water t...
Questions







Chemistry, 31.03.2020 04:04




English, 31.03.2020 04:04

Social Studies, 31.03.2020 04:04

Advanced Placement (AP), 31.03.2020 04:04





Mathematics, 31.03.2020 04:05