
Chemistry, 20.09.2020 05:01 daniel9299
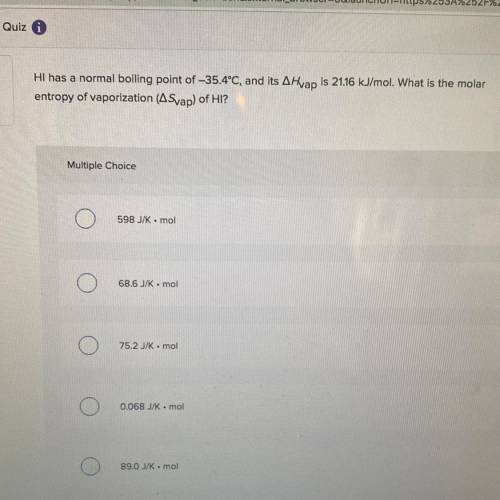
Hi has a normal boiling point of -35.4°C, and its A Hvap is 21.16 kJ/mol. What is the molar
entropy of vaporization (Svap) of HI?
Multiple Choice
598 JK-mol
68.6 JK . mol
752 kmol
0.068 J/K mol
89.0 J/K mol

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1
You know the right answer?
Hi has a normal boiling point of -35.4°C, and its A Hvap is 21.16 kJ/mol. What is the molar
entropy...
Questions

Biology, 13.09.2021 09:10

Geography, 13.09.2021 09:10

History, 13.09.2021 09:10

Social Studies, 13.09.2021 09:10

Mathematics, 13.09.2021 09:10

History, 13.09.2021 09:10


Mathematics, 13.09.2021 09:10





Mathematics, 13.09.2021 09:10

Mathematics, 13.09.2021 09:10

Mathematics, 13.09.2021 09:10

Mathematics, 13.09.2021 09:10

Mathematics, 13.09.2021 09:10

Mathematics, 13.09.2021 09:10


Mathematics, 13.09.2021 09:10



