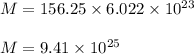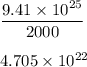Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

You know the right answer?
If you dissolve 5.00 kg of methanol (CH_4O) in 2.00 m^3 of water, how many molecules of methanol per...
Questions

Chemistry, 29.07.2021 05:20

Physics, 29.07.2021 05:20

Advanced Placement (AP), 29.07.2021 05:20



Social Studies, 29.07.2021 05:20





Mathematics, 29.07.2021 05:20

Mathematics, 29.07.2021 05:20


Law, 29.07.2021 05:20





Physics, 29.07.2021 05:20

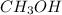 ) , m = 5 kg = 5000 g .
) , m = 5 kg = 5000 g .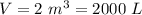 .
.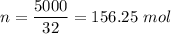 .
.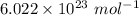 .
.