
Chemistry, 20.09.2020 09:01 imalexiscv
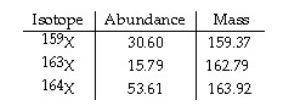
The element X has three naturally occurring isotopes. The isotopic masses (amu) and % abundances of the isotopes are given in the table below. Calculate the average atomic mass of the element in amu. PLEASE HELP ASAP

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1


Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3
You know the right answer?
The element X has three naturally occurring isotopes. The isotopic masses (amu) and % abundances of...
Questions




English, 05.05.2020 07:49

Biology, 05.05.2020 07:49

English, 05.05.2020 07:49





English, 05.05.2020 07:49

Mathematics, 05.05.2020 07:49


Mathematics, 05.05.2020 07:49

Mathematics, 05.05.2020 07:49


Mathematics, 05.05.2020 07:49


Mathematics, 05.05.2020 07:49

Mathematics, 05.05.2020 07:49



