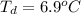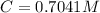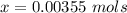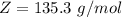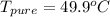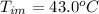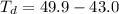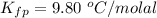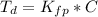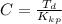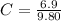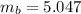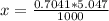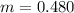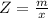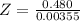Chemistry, 20.09.2020 09:01 davidleew24
Benzophenone freezing point= 49.9 C Benzophenone + unknown freezing point= 43.0 C Benzophenone mass= 5.047 g Unknown mass= .480 g 1. From the difference between the freezing points of the pure benzophenone and the unknown + benzophenone solution, calculate the freezing point depression of the solution. 2. Given the freezing point depression constant for benzophenone, Kfp= 9.80 C/molal, calculate the molality of the solution of unknown in benzophenone. (answer in m) 3. Now use the calculated value for the molality of the solution and the mass of the benzophenone to compute the number of moles of solute present in the solution. 4. Use that calculated number of moles and mass of solute to determine the approximate molar mass (Gram molecular weight) of the unknown solute. (answer in g/mol)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2


Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1
You know the right answer?
Benzophenone freezing point= 49.9 C Benzophenone + unknown freezing point= 43.0 C Benzophenone mass=...
Questions


Social Studies, 09.09.2021 23:00

Mathematics, 09.09.2021 23:00



Mathematics, 09.09.2021 23:00

History, 09.09.2021 23:00




World Languages, 09.09.2021 23:00


Spanish, 09.09.2021 23:00

English, 09.09.2021 23:00



Social Studies, 09.09.2021 23:00



History, 09.09.2021 23:00