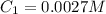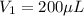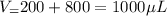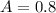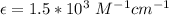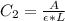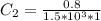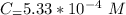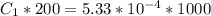Chemistry, 20.09.2020 17:01 haydenamrhein1693
In a laboratory experiment, you are asked to determine the molar concentration of a solution of an unknown compound, X. The solution diluted in with water (200 µL of X + 800 µL of H2O) has an absorbance at 425 nm of 0.8 and a molar extinction coefficient of 1.5 x103 M-1cm-1 at 425 nm. What is the molar concentration of the original solution of X?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:40
Does the energy in a solid increase or decrease when changing to a liquid?
Answers: 1

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
You know the right answer?
In a laboratory experiment, you are asked to determine the molar concentration of a solution of an u...
Questions

Mathematics, 23.04.2021 20:30



Mathematics, 23.04.2021 20:30

Social Studies, 23.04.2021 20:30

Chemistry, 23.04.2021 20:30



Mathematics, 23.04.2021 20:30



History, 23.04.2021 20:30

Mathematics, 23.04.2021 20:30

Mathematics, 23.04.2021 20:30



Mathematics, 23.04.2021 20:30

History, 23.04.2021 20:30

Mathematics, 23.04.2021 20:30

Mathematics, 23.04.2021 20:30