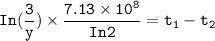Chemistry, 20.09.2020 16:01 joannachavez12345
Since the half-life of 235U (7. 13 x 108 years) is less than that of 238U (4.51 x 109 years), the isotopic abundance of 235U has been steadily decreasing since the earth was fonned about 4.5 billion years ago. How long ago was the isotopic abundance of 235U equal to 3.0 a/o, the enrichment of the uranium used in many nuclear power plants

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
Since the half-life of 235U (7. 13 x 108 years) is less than that of 238U (4.51 x 109 years), the is...
Questions




















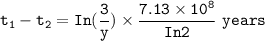
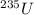 =
= 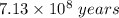 is less than that of
is less than that of 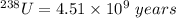
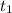
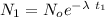
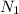 = Number of radioactive atoms relating to the weight of y of
= Number of radioactive atoms relating to the weight of y of 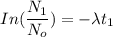
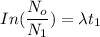 --- (1)
--- (1)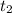
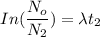 ---- (2)
---- (2)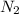 = Number of radioactive atoms of
= Number of radioactive atoms of 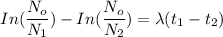
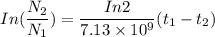 ( since
( since 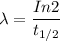 )
)