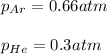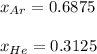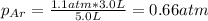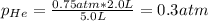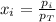Chemistry, 20.09.2020 15:01 noeliaortiz3478
Two containers, one with a volume of 3.0 L and the other with a volume of 2.0 L contain, respectively, argon gas at 1.1 atm and helium at 0.75 atm. The containers are initially separated by a valve, and then the valve is opened to connect the two containers. Assume perfect gases and determine the followings.
a. The total pressure of the mixed gases
b. The partial pressure of each gas
c. The mole fraction of each gas

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3


Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3
You know the right answer?
Two containers, one with a volume of 3.0 L and the other with a volume of 2.0 L contain, respectivel...
Questions




Mathematics, 31.07.2020 04:01

Chemistry, 31.07.2020 04:01

Mathematics, 31.07.2020 04:01

Mathematics, 31.07.2020 04:01





Chemistry, 31.07.2020 04:01



Mathematics, 31.07.2020 04:01





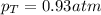 .
.