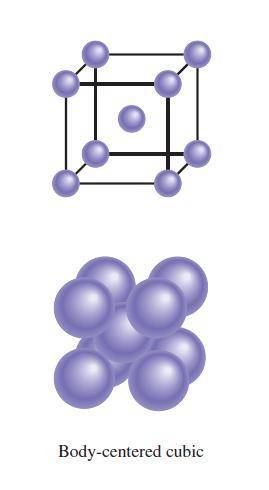
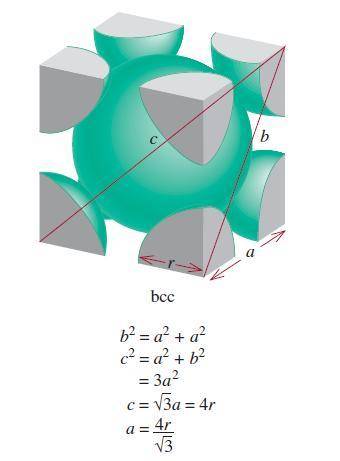
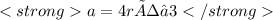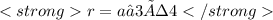A certain metal crystallizes in a lattice described by a body-centered cubic (bcc) unit cell. The lattice constant has been measured by X-ray crystallography to be . Calculate the radius of an atom of . Be sure your answer has significant digits, and be sure it has the correct unit symbol.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1


Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
You know the right answer?
A certain metal crystallizes in a lattice described by a body-centered cubic (bcc) unit cell. The la...
Questions


Biology, 04.08.2019 14:00

Mathematics, 04.08.2019 14:00

Biology, 04.08.2019 14:00

Social Studies, 04.08.2019 14:00




Mathematics, 04.08.2019 14:00








Mathematics, 04.08.2019 14:00


Mathematics, 04.08.2019 14:00

Mathematics, 04.08.2019 14:00