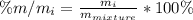Chemistry, 20.09.2020 18:01 kaylaelaine18
A student was provided with a solid sample made up of a mixture of components. Prior to separation, the student measured 2.895 g of the mixture. After separation, the student found the mixture contained the following four components:
Component 1: 1.12g
Component 2: 0.756g
Component 3: 0.254g
Component 4: 0.525g
Which component has the highest mass % for the mixture?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
You know the right answer?
A student was provided with a solid sample made up of a mixture of components. Prior to separation,...
Questions


Mathematics, 11.10.2020 19:01


Mathematics, 11.10.2020 19:01


Mathematics, 11.10.2020 19:01



English, 11.10.2020 19:01



Biology, 11.10.2020 19:01


Chemistry, 11.10.2020 19:01

Mathematics, 11.10.2020 19:01




Mathematics, 11.10.2020 19:01