
Chemistry, 20.09.2020 19:01 lucyamine0
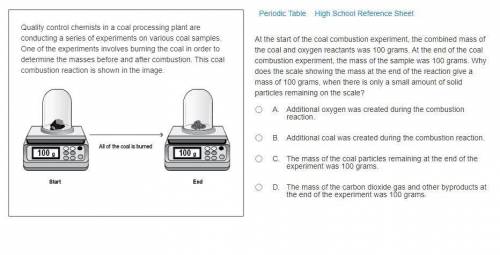
At the start of the coal combustion experiment, the combined mass of the coal and oxygen reactants was 100 grams. At the end of the coal combustion experiment, the mass of the sample was 100 grams. Why does the scale showing the mass at the end of the reaction give a mass of 100 grams, when there is only a small amount of solid particles remaining on the scale?
A. Additional oxygen was created during the combustion reaction.
B. Additional coal was created during the combustion reaction.
C. The mass of the coal particles remaining at the end of the experiment was 100 grams.
D. The mass of the carbon dioxide gas and other byproducts at the end of the experiment was 100 grams.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
At the start of the coal combustion experiment, the combined mass of the coal and oxygen reactants w...
Questions


Chemistry, 16.02.2021 21:20

Mathematics, 16.02.2021 21:20

Mathematics, 16.02.2021 21:20

Mathematics, 16.02.2021 21:20

Mathematics, 16.02.2021 21:20


Mathematics, 16.02.2021 21:20


Mathematics, 16.02.2021 21:20

Arts, 16.02.2021 21:20

Mathematics, 16.02.2021 21:20

Mathematics, 16.02.2021 21:20

Mathematics, 16.02.2021 21:20


History, 16.02.2021 21:20

History, 16.02.2021 21:20


Physics, 16.02.2021 21:20




