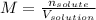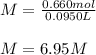Chemistry, 20.09.2020 20:01 tamyahamlin02p6b7yt
0.660 moles of NaCl are dissolved in 95.0 mL of water. Calculate the molarity of the NaCl solution. A) 62.7 M B) 0.069 M C) 6.95 M D) 0.0069 M

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1
You know the right answer?
0.660 moles of NaCl are dissolved in 95.0 mL of water. Calculate the molarity of the NaCl solution....
Questions

English, 16.06.2021 20:00

Mathematics, 16.06.2021 20:00

Chemistry, 16.06.2021 20:00

Social Studies, 16.06.2021 20:00

Mathematics, 16.06.2021 20:00

Physics, 16.06.2021 20:00

English, 16.06.2021 20:00

Mathematics, 16.06.2021 20:00



Mathematics, 16.06.2021 20:00

Mathematics, 16.06.2021 20:00


History, 16.06.2021 20:00

Geography, 16.06.2021 20:00

History, 16.06.2021 20:00

Mathematics, 16.06.2021 20:00

English, 16.06.2021 20:00