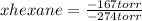A solution contains a mixture of pentane and hexane at room temperature. The solution has a vapor pressure of 258 torr . Pure pentane and hexane have vapor pressures of 425 torr and 151 torr, respectively, at room temperature. What is the mole fraction of hexane? (Assume ideal behavior.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

You know the right answer?
A solution contains a mixture of pentane and hexane at room temperature. The solution has a vapor pr...
Questions

Biology, 22.01.2021 05:50



History, 22.01.2021 05:50

English, 22.01.2021 05:50

Chemistry, 22.01.2021 05:50

Computers and Technology, 22.01.2021 05:50

Mathematics, 22.01.2021 05:50


History, 22.01.2021 05:50



Mathematics, 22.01.2021 05:50





Mathematics, 22.01.2021 05:50


Chemistry, 22.01.2021 05:50