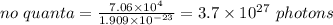Chemistry, 21.09.2020 09:01 mrmendrala
Heating 235 g of water from 22.6°C to 94.4°C in a microwave oven requires 7.06 × 104 J of energy. If the microwave frequency is 2.88 × 1010 s−1, how many quanta are required to supply the 7.06 × 104 J? The value for Planck's constant is 6.63 × 10-34 Jᐧs/quantum. The formula to use is Energy / (Planck's constant x Frequency).

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

You know the right answer?
Heating 235 g of water from 22.6°C to 94.4°C in a microwave oven requires 7.06 × 104 J of energy. If...
Questions


Mathematics, 23.04.2021 18:10

Mathematics, 23.04.2021 18:10


Mathematics, 23.04.2021 18:10


Mathematics, 23.04.2021 18:10


History, 23.04.2021 18:10

Social Studies, 23.04.2021 18:10


Mathematics, 23.04.2021 18:10


Computers and Technology, 23.04.2021 18:10



Mathematics, 23.04.2021 18:10

Chemistry, 23.04.2021 18:10

French, 23.04.2021 18:10

Mathematics, 23.04.2021 18:10