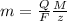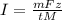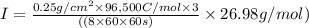The weight loss of an aluminum (Al) alloy corroding in HCI acid was observed to be 0.250 g/cm2 after an 8 h immersion period. What is the corresponding corrosion current density in mA/em2, assuming that all the corrosion is due to the reaction:
Al → Al3+ + 3e
The atomic weight of Al is 26.98 g/mol.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 294 k. if the balloon is cooled to 277 k and its volume decreased to 1 l, what will the new pressure in the balloon be?
Answers: 1

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3
You know the right answer?
The weight loss of an aluminum (Al) alloy corroding in HCI acid was observed to be 0.250 g/cm2 after...
Questions


Health, 09.10.2019 00:50

Social Studies, 09.10.2019 00:50

Biology, 09.10.2019 00:50


Business, 09.10.2019 00:50




Biology, 09.10.2019 00:50

Geography, 09.10.2019 00:50

Physics, 09.10.2019 00:50

Mathematics, 09.10.2019 00:50

Mathematics, 09.10.2019 00:50



Mathematics, 09.10.2019 00:50

History, 09.10.2019 00:50

Physics, 09.10.2019 00:50

Geography, 09.10.2019 00:50