
Chemistry, 22.09.2020 20:01 leannamat2106
A pair of 100mL samples of water are taken from a well bored into a large underground salt NaCl deposit. Sample #1 is from the top of the well, and is initially at 32°C. Sample #2 is from a depth of 50.m, and is initially at 42°C. Both samples are allowed to come to room temperature (20.°C) and 1atm pressure. An NaCl precipitate is seen to form in Sample #1.
a. A bigger mass of NaCl precipitate will form in Sample
b. A smaller mass of NaCl precipitate will form in Sample
c. The same mass of NaCl precipitate will form in Sample #2.
d. No, precipitate will form in Sample #2.
e. I need more information to predict whether and how much precipitate will form in Sample #2.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2


Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
A pair of 100mL samples of water are taken from a well bored into a large underground salt NaCl depo...
Questions




















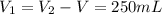
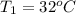
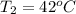
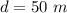
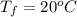
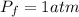
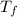 there will be a greater decrease in temperature in sample 2 than in sample 1 so more precipitate of NaCl will be formed in sample 2 than in sample one
there will be a greater decrease in temperature in sample 2 than in sample 1 so more precipitate of NaCl will be formed in sample 2 than in sample one 

