
Chemistry, 23.09.2020 01:01 haydenamrhein1693
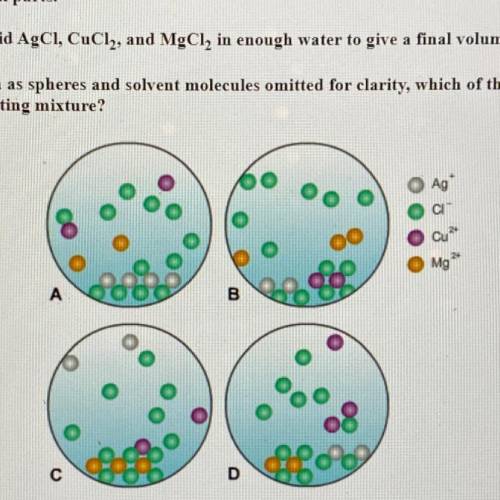
A chemist mixes solid AgCl, CuCl2, and MgCl2, in enough water to give a final volume of 50.0 mL.
(a) With ions shown as spheres and solvent molecules omitted for clarity, which of the following best
represents the resulting mixture?
(b) If each sphere represents 5.5*10^-3 mol of ions, what is the total concentration of dissolved (separated) ions?
(c) What is the total mass of the solid?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:00
Based on the position vs. time graph which velocity vs. time graph would correspond to the data
Answers: 2

Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3

Chemistry, 23.06.2019 08:30
Kelly has come up with an explanation for why her sister is sometimes in a good mood and other times in a bad mood. she speculates that it is based on the hours of sleep her sister got the previous night. this explanation for her sister's behaviors is an example of a(n)
Answers: 3
You know the right answer?
A chemist mixes solid AgCl, CuCl2, and MgCl2, in enough water to give a final volume of 50.0 mL.
(a...
Questions








Health, 25.09.2019 08:00

Mathematics, 25.09.2019 08:00

Mathematics, 25.09.2019 08:00



Social Studies, 25.09.2019 08:00




Mathematics, 25.09.2019 08:00

Mathematics, 25.09.2019 08:00

Mathematics, 25.09.2019 08:00




