
Chemistry, 23.09.2020 14:01 tiwaribianca475
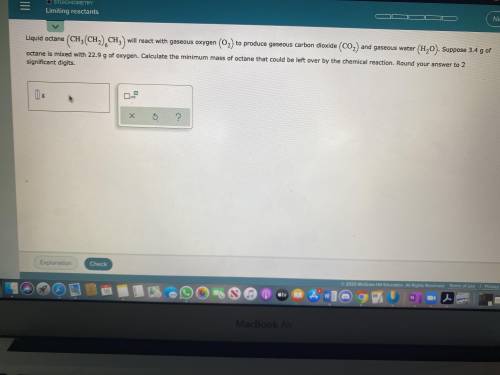
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water. Suppose 3.4g of octane is mixed with 22.9g of oxygen. Calculate the minimum mass of octane that could be left over by the chemical reaction. Round your answer to 2 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2
You know the right answer?
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water. Su...
Questions

Mathematics, 14.06.2021 16:40

Mathematics, 14.06.2021 16:40




Physics, 14.06.2021 16:50



Mathematics, 14.06.2021 16:50



Arts, 14.06.2021 16:50





Mathematics, 14.06.2021 16:50

Chemistry, 14.06.2021 16:50


Mathematics, 14.06.2021 16:50



