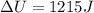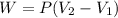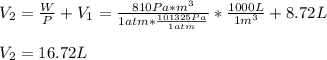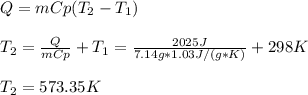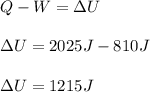Chemistry, 23.09.2020 18:01 natalie2sheffield
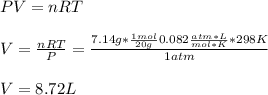
A system is composed of 7.14 grams of Ne gas at 298 K and 1 atm. When 2025 joules of heat are added to the system at constant pressure, the resultant expansion causes the system to perform 810 joules of work. Calculate the following. (a) The initial state variables (P, V, T) (b) The final state variables. (c) The change in internal energy for the process. The molecular weight of Ne is 20 and you can assume Ne behaves as an ideal gas. (

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1


Chemistry, 23.06.2019 11:50
It takes 155. kj/mol to break a fluorine-fluorine single bond. calculate the maximum wavelength of light for which a flouine-flouring single bond could be broken by absorbing a single photon
Answers: 1
You know the right answer?
A system is composed of 7.14 grams of Ne gas at 298 K and 1 atm. When 2025 joules of heat are added...
Questions

English, 04.08.2019 10:00



Mathematics, 04.08.2019 10:00

History, 04.08.2019 10:00


Mathematics, 04.08.2019 10:00

History, 04.08.2019 10:00

Mathematics, 04.08.2019 10:00

Business, 04.08.2019 10:00

Business, 04.08.2019 10:00





Mathematics, 04.08.2019 10:00

Mathematics, 04.08.2019 10:00

Mathematics, 04.08.2019 10:00