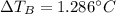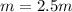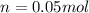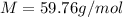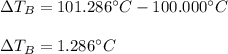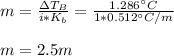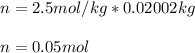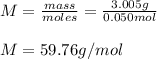Suppose 3.005 g of a nonvolatile solute is added to 20.02 g of water (the solvent), and the boiling point increases from 100.000 OC to 101.286 OC. Determine the TB, molality, moles, and molecular weight for the solute if kb for water is 0.512 OC/m. Report each value using the correct number of significant digits. Refer to Example 1.2 and pages 3-4 in the chapter 1 notes for general chemistry 1 to understand significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
Suppose 3.005 g of a nonvolatile solute is added to 20.02 g of water (the solvent), and the boiling...
Questions


Mathematics, 20.02.2021 05:40

Physics, 20.02.2021 05:40

Mathematics, 20.02.2021 05:40



Mathematics, 20.02.2021 05:40

Mathematics, 20.02.2021 05:40

Business, 20.02.2021 05:40



Mathematics, 20.02.2021 05:40



Mathematics, 20.02.2021 05:40

Mathematics, 20.02.2021 05:40



Health, 20.02.2021 05:40

Mathematics, 20.02.2021 05:40