
Chemistry, 18.09.2019 21:40 carlosiscr7
Capsaicin, the compound that gives the hot taste to chili peppers, has the formula c18h27no3. its molar mass is 305.42 g/mol. calculate the mass percent of each element in the compound.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
Capsaicin, the compound that gives the hot taste to chili peppers, has the formula c18h27no3. its mo...
Questions

Mathematics, 23.10.2019 02:00

History, 23.10.2019 02:00




Mathematics, 23.10.2019 02:00

Physics, 23.10.2019 02:00



Mathematics, 23.10.2019 02:00


English, 23.10.2019 02:00


English, 23.10.2019 02:00



Mathematics, 23.10.2019 02:00

Mathematics, 23.10.2019 02:00


Chemistry, 23.10.2019 02:00

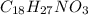 is an organic compound with a nitro group present in it.
is an organic compound with a nitro group present in it.

