
Chemistry, 24.09.2020 15:01 yungking1329
Calculate the molar volume occupied by 1 mole of N2 using the van der Waals equation in the form of virial expansion at (a) its critical temperature and (b) its Boyle temperature. Assume that the pressure is 10 atm throughout. At what temperature is the gas most perfect? Use the following data: Tc = 126.3 K, a=1.352 L2 atm mol-2, b = 0.0387 L mol-1

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
Calculate the molar volume occupied by 1 mole of N2 using the van der Waals equation in the form of...
Questions




Mathematics, 11.06.2021 01:00


Mathematics, 11.06.2021 01:00

Biology, 11.06.2021 01:00


Biology, 11.06.2021 01:00

English, 11.06.2021 01:00

Mathematics, 11.06.2021 01:00

Biology, 11.06.2021 01:00

Mathematics, 11.06.2021 01:00







Mathematics, 11.06.2021 01:00

 = 1 mole
= 1 mole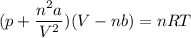
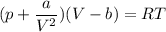
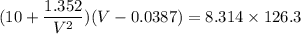
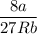
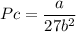
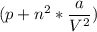 depending on the circumstances.
depending on the circumstances.

