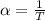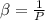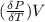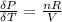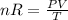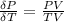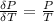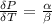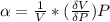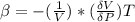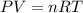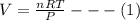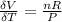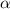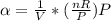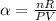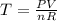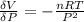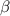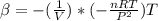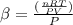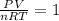The coefficient of thermal expansion α = (1/V)(∂V/∂T)p. Using the equation of state, compute the value of α for an ideal gas. The coefficient of compressibility β is define by β = -(1/V)(∂V/∂p)T. Compute the value of β for an ideal gas. For an ideal gas, express the derivative (∂p/∂T)v in terms of α and β. Do the same derivative for van der Waals gas.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1



Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
You know the right answer?
The coefficient of thermal expansion α = (1/V)(∂V/∂T)p. Using the equation of state, compute the val...
Questions





Mathematics, 03.09.2020 18:01



Mathematics, 03.09.2020 18:01


Mathematics, 03.09.2020 18:01



Geography, 03.09.2020 18:01

English, 03.09.2020 18:01

English, 03.09.2020 18:01



Mathematics, 03.09.2020 18:01

History, 03.09.2020 18:01