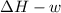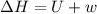Chemistry, 24.09.2020 21:01 MalikaJones
One mole of liquid water at 100°C is heated until the liquid is converted entirely to vapor at 100°C and 1 bar pressure. Calculate q, w, U, and H for each of the following:
A. The vaporization is carried out in a cylinder where the external pressure on the piston is maintained at 1 bar throughout.
B. The cylinder is first expanded against a vacuum (Pex=0) to the same volumn as in part (a), and then sufficient heat is added to vaporize the liquid completely to 1 atm pressure.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
One mole of liquid water at 100°C is heated until the liquid is converted entirely to vapor at 100°C...
Questions






Mathematics, 04.07.2019 17:50

Mathematics, 04.07.2019 17:50

Advanced Placement (AP), 04.07.2019 17:50


Social Studies, 04.07.2019 17:50




Biology, 04.07.2019 17:50


Biology, 04.07.2019 17:50



History, 04.07.2019 17:50

Health, 04.07.2019 17:50

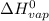 = 2260 kJ/kg
= 2260 kJ/kg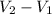 )
)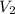 = specific volume of steam = 0.0305 m³/mol
= specific volume of steam = 0.0305 m³/mol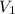 = specific volume of water = 0.00001837 m³/mol
= specific volume of water = 0.00001837 m³/mol 3.05 kJ
3.05 kJ