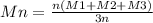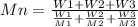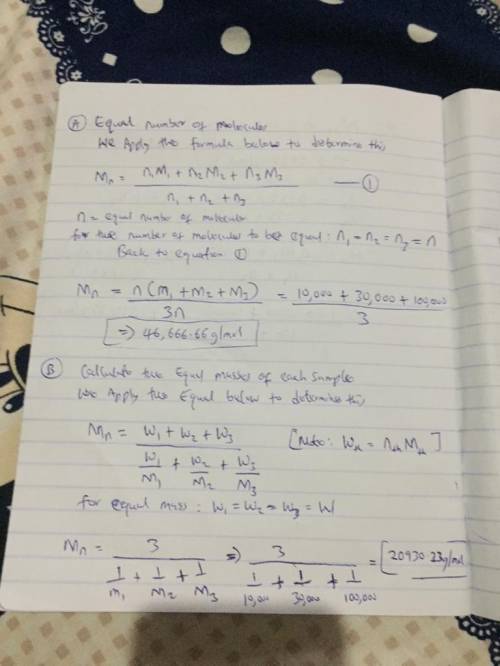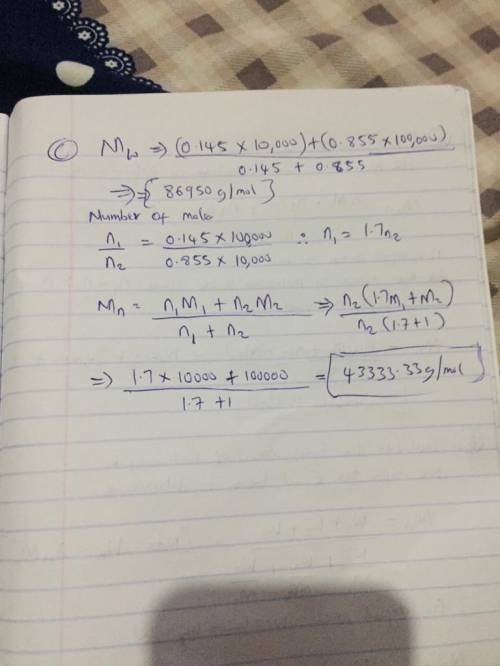Chemistry, 24.09.2020 22:01 savagesquid4807
Two mixtures were prepared from three very narrow molar mass distribution polystyrene samples with molar masses of 10,000, 30,000 and 100,000 g/mola indicated below: (A) Equal numbers of molecules of each sample (B) Equal masses of each sample. For each of the mixtures, calculate the number-average and weight-average molar masses and comment upon the meaning of the values.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

You know the right answer?
Two mixtures were prepared from three very narrow molar mass distribution polystyrene samples with m...
Questions




Geography, 27.02.2020 21:59

English, 27.02.2020 21:59


English, 27.02.2020 21:59



English, 27.02.2020 21:59









English, 27.02.2020 21:59

Chemistry, 27.02.2020 21:59