

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1


You know the right answer?
What would happen if N2 were added to N2(g) + O2(g) = 2NO(9) at
equilibrium?
O A. Keq w...
O A. Keq w...
Questions

History, 10.10.2021 14:00


Chemistry, 10.10.2021 14:00

Chemistry, 10.10.2021 14:00


Arts, 10.10.2021 14:00

English, 10.10.2021 14:00

SAT, 10.10.2021 14:00

Mathematics, 10.10.2021 14:00

Health, 10.10.2021 14:00

Computers and Technology, 10.10.2021 14:00



Mathematics, 10.10.2021 14:00

Biology, 10.10.2021 14:00

English, 10.10.2021 14:00



History, 10.10.2021 14:00

Mathematics, 10.10.2021 14:00

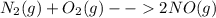
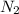 is added .
is added .

