Chemistry, 25.09.2020 02:01 brookeanne723
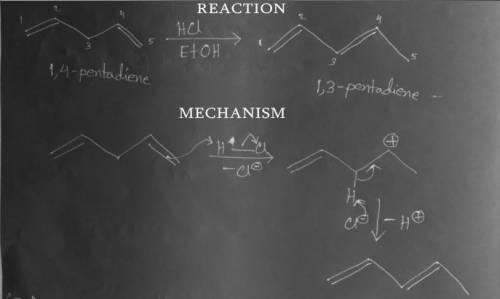
Upon treatment with a catalytic amount of an acid such as HCl in ethanol, 1,4-pentadiene undergoes rearrangement to an isomer that is more stable than the starting compound. Draw the structure of this isomer and the mechanism by which it is formed. Use the curved arrow formalism to depict all bond-forming and bond-breaking steps, and show all intermediates.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1
You know the right answer?
Upon treatment with a catalytic amount of an acid such as HCl in ethanol, 1,4-pentadiene undergoes r...
Questions

Spanish, 07.11.2020 04:40

History, 07.11.2020 04:40



English, 07.11.2020 04:40

Mathematics, 07.11.2020 04:40

Mathematics, 07.11.2020 04:40

Health, 07.11.2020 04:40

Chemistry, 07.11.2020 04:40

History, 07.11.2020 04:40


Mathematics, 07.11.2020 04:40


English, 07.11.2020 04:40

Mathematics, 07.11.2020 04:40




Mathematics, 07.11.2020 04:40