
Chemistry, 25.09.2020 09:01 rachel63892
LOL please help me ✨
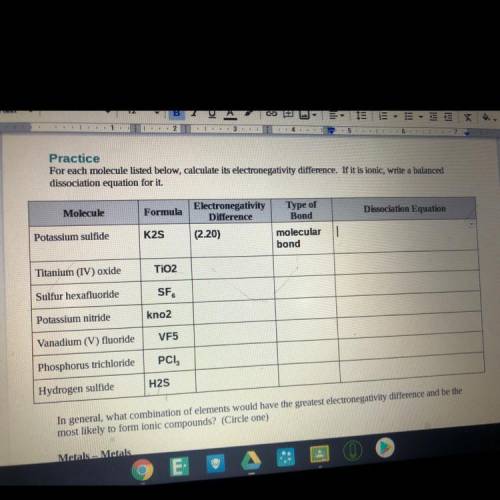
For each molecule listed below, calculate its electronegativity difference. If it is ionic, write a balanced
dissociation equation for it
Dissociation Equation
Molecule
Formula
Electronegativity
Difference
(2.20)
Type of
Bond
molecular
bond
Potassium sulfide
K2S
Titanium (IV) oxide
TiO2
1
Sulfur hexafluoride
SF
Potassium nitride
kno2
Vanadium (V) fluoride
VF5
Phosphorus trichloride
PCI,
Hydrogen sulfide
H2S

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 23.06.2019 03:30
In general metals get as you move from left to right across the periodic table.
Answers: 1

Chemistry, 23.06.2019 05:30
Suppose you discovered a new element with 120 protons and 2 electrons in its outer level . i'm what group does this new element belong? what properties would you expect it to have
Answers: 1
You know the right answer?
LOL please help me ✨
For each molecule listed below, calculate its electronegativity difference. If...
Questions

Mathematics, 21.12.2021 04:50


English, 21.12.2021 04:50

French, 21.12.2021 04:50

Business, 21.12.2021 04:50


Computers and Technology, 21.12.2021 04:50




Social Studies, 21.12.2021 04:50

Physics, 21.12.2021 04:50










