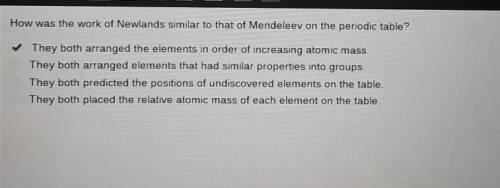
How was the work of Newlands similar to that of Mendeleev on the periodic table?
They both arranged the elements in order of increasing atomic mass.
They both arranged elements that had similar properties into groups.
They both predicted the positions of undiscovered elements on the table.
They both placed the relative atomic mass of each element on the table.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2


Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
How was the work of Newlands similar to that of Mendeleev on the periodic table?
They both arranged...
Questions








Mathematics, 21.02.2020 04:12



Mathematics, 21.02.2020 04:12

English, 21.02.2020 04:12





Chemistry, 21.02.2020 04:12

Mathematics, 21.02.2020 04:12