Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2
You know the right answer?
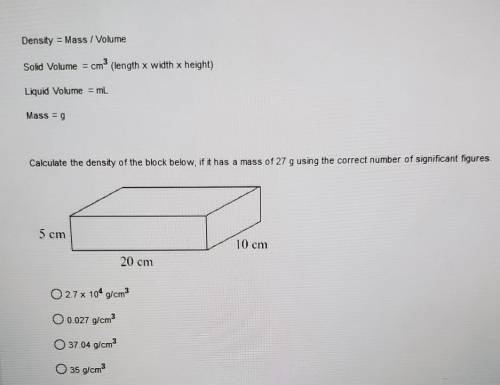
Calculate the density of the block below, if it has a mass of 27 g using the correct number of signi...
Questions




Spanish, 25.03.2021 09:50

Chemistry, 25.03.2021 09:50

Mathematics, 25.03.2021 09:50


Mathematics, 25.03.2021 09:50

Mathematics, 25.03.2021 09:50

Biology, 25.03.2021 09:50

Business, 25.03.2021 09:50

Mathematics, 25.03.2021 09:50

History, 25.03.2021 09:50

Mathematics, 25.03.2021 09:50

Mathematics, 25.03.2021 09:50




Social Studies, 25.03.2021 09:50