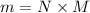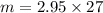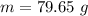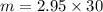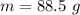Chemistry, 02.10.2020 17:01 trintrin227
Hydrogen cyanide, HCN, can be made by a two-step process. First, ammonia reacts with O2 to give nitric oxide, NO.
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
Then nitric oxide reacts with methane, CH4.
2NO(g) + 2CH4(g) → 2HCN(g) + 2H2O(g) + H2(g)
When 50.2 g of ammonia and 48.4 g of methane are used, how many grams of hydrogen cyanide can be produced? How many grams of which reactant remain at the end of both reactions? (You may assume that O2 is in excess in the first reaction.)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1

Chemistry, 23.06.2019 09:00
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1
You know the right answer?
Hydrogen cyanide, HCN, can be made by a two-step process. First, ammonia reacts with O2 to give nitr...
Questions


Mathematics, 12.01.2021 05:00


Mathematics, 12.01.2021 05:00

Mathematics, 12.01.2021 05:00



Geography, 12.01.2021 05:00

Mathematics, 12.01.2021 05:00



Physics, 12.01.2021 05:00


Biology, 12.01.2021 05:00



Business, 12.01.2021 05:00

English, 12.01.2021 05:00

Mathematics, 12.01.2021 05:00

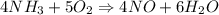
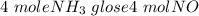
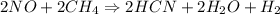
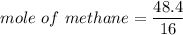
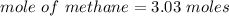
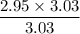 = 2.95 mol HCN
= 2.95 mol HCN