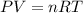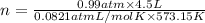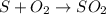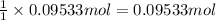Chemistry, 14.10.2019 21:40 shardonnay2160
What mass of sulfur has to burn to produce 4.5l so2 at 300°c and 101 kpa in the following reaction?
a. 13.5 g s
b. 3.07 g s
c. 68.8 g s
d. 41.0 g s

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
What mass of sulfur has to burn to produce 4.5l so2 at 300°c and 101 kpa in the following reaction?...
Questions

Social Studies, 02.09.2020 02:01



Computers and Technology, 02.09.2020 02:01










History, 02.09.2020 02:01

Mathematics, 02.09.2020 02:01

Biology, 02.09.2020 02:01

English, 02.09.2020 02:01

Mathematics, 02.09.2020 02:01