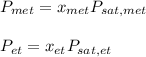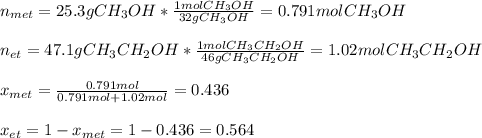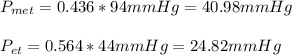Chemistry, 04.10.2020 14:01 melissakm77
ure to answer all parts. The vapor pressure of ethanol (C2H5OH) at 20°C is 44 mmHg, and the vapor pressure of methanol (CH3OH) at the same temperature is 94 mmHg. A mixture of 25.3 g of methanol and 47.1 g of ethanol is prepared and can be assumed to behave as an ideal solution. Calculate the vapor pressure of methanol and ethanol above this solution at 20°C. Be sure to report your answers to the correct number of significant figures.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

You know the right answer?
ure to answer all parts. The vapor pressure of ethanol (C2H5OH) at 20°C is 44 mmHg, and the vapor pr...
Questions




Mathematics, 03.04.2020 02:59

Biology, 03.04.2020 02:59

Mathematics, 03.04.2020 03:00

Mathematics, 03.04.2020 03:00


Mathematics, 03.04.2020 03:00


History, 03.04.2020 03:00




Mathematics, 03.04.2020 03:00

Mathematics, 03.04.2020 03:00

Mathematics, 03.04.2020 03:00


Chemistry, 03.04.2020 03:00