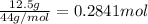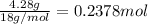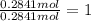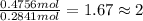Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
Complete combustion of 3.90 g of a hydrocarbon produced 12.5 g of co2 and 4.28 g of h2o. what is the...
Questions



Mathematics, 30.11.2021 17:20

Mathematics, 30.11.2021 17:20


Mathematics, 30.11.2021 17:20





History, 30.11.2021 17:20

Biology, 30.11.2021 17:20

Mathematics, 30.11.2021 17:20

Chemistry, 30.11.2021 17:20

Mathematics, 30.11.2021 17:20



English, 30.11.2021 17:20


Computers and Technology, 30.11.2021 17:20

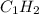 .
.