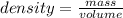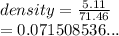Chemistry, 05.10.2020 15:01 borgesalfonso12
A block of metal has a mass of 51.11 g and displaces 71.46 ml of water. Calculate the density of the metal in g/ml.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:20
Both 1,2−dihydronaphthalene and 1,4−dihydronaphthalene may be selectively hydrogenated to 1,2,3,4−tetrahydronaphthalene. one of these isomers has a heat of hydrogenation of 101 kj/mol (24.1 kcal/mol), and the heat of hydrogenation of the other is 113 kj/mol (27.1 kcal/mol). match the heat of hydrogenation with the appropriate dihydronaphthalene.
Answers: 2

Chemistry, 21.06.2019 15:30
If 200.0g of copper(ll) sulfate react with an excess of zinc metal, what is the theoretical yield of copper
Answers: 1

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3
You know the right answer?
A block of metal has a mass of 51.11 g and displaces 71.46 ml of water. Calculate the density of the...
Questions

History, 10.10.2021 08:40

Mathematics, 10.10.2021 08:40



Mathematics, 10.10.2021 08:40


History, 10.10.2021 08:40


English, 10.10.2021 08:40

Biology, 10.10.2021 08:40


Geography, 10.10.2021 08:40




Chemistry, 10.10.2021 08:40

Mathematics, 10.10.2021 08:40



Mathematics, 10.10.2021 08:40