Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

You know the right answer?
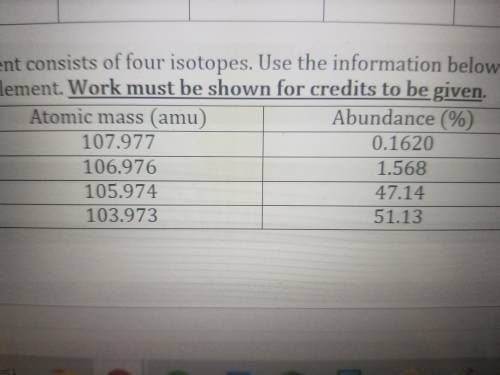
A hypothetical element consists of four isotopes. Use the information below to calculate the average...
Questions

Physics, 19.08.2019 21:30



History, 19.08.2019 21:30



Social Studies, 19.08.2019 21:30

Social Studies, 19.08.2019 21:30



Mathematics, 19.08.2019 21:30

Geography, 19.08.2019 21:30



Mathematics, 19.08.2019 21:30


Social Studies, 19.08.2019 21:30


Mathematics, 19.08.2019 21:30