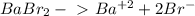Chemistry, 15.10.2019 04:30 DEVORIA2001
Determine the concentrations of babr2, ba2 , and br– in a solution prepared by dissolving 2.37 × 10–4 g babr2 in 1.00 l of water. express all three concentrations in molarity. additionally, express the concentrations of the ionic species in parts per million (ppm).

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
You know the right answer?
Determine the concentrations of babr2, ba2 , and br– in a solution prepared by dissolving 2.37 × 10–...
Questions


Mathematics, 12.03.2021 21:30

Geography, 12.03.2021 21:30


Mathematics, 12.03.2021 21:30


Mathematics, 12.03.2021 21:30


Mathematics, 12.03.2021 21:30



Chemistry, 12.03.2021 21:30

Physics, 12.03.2021 21:30


Mathematics, 12.03.2021 21:30


Mathematics, 12.03.2021 21:30

Biology, 12.03.2021 21:30


Mathematics, 12.03.2021 21:30