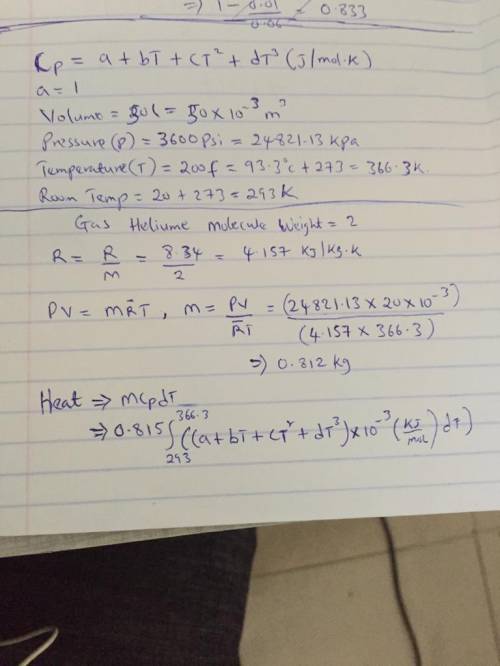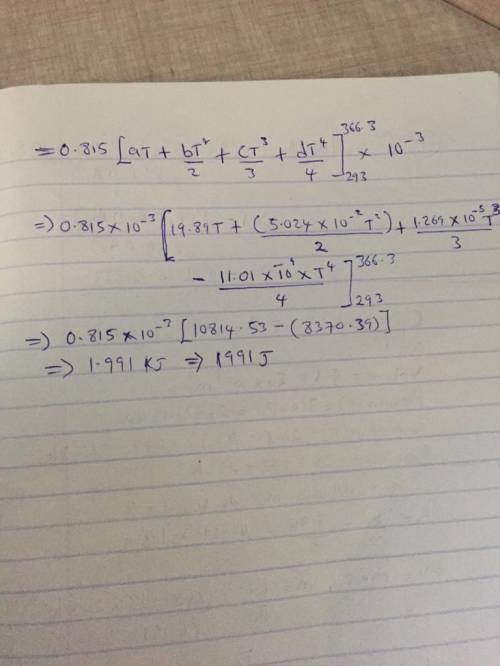The specific heats at constant pressure of some common gases are provided as a thirdorder polynomial: �;<<< = � + �� + ��C + ��E, with units J/(mol K). For methane (CH4) the coefficients are � = 19.89, � = 5.024 × 10NC, � = 1.269 × 10NO, � = −11.01 × 10NQ. At a production facility, the gas in a 50-liter tank is compressed to 3,600 psi (gage) during which time the temperature rises to 200°F. How much heat in J is given off as the gas cools to room temperature? Suppose the compressed gas is helium, how much heat would be given off in this case?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
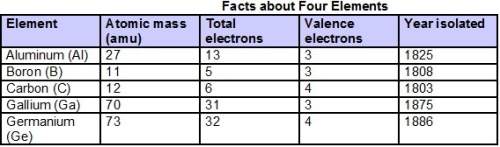
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3


Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1
You know the right answer?
The specific heats at constant pressure of some common gases are provided as a thirdorder polynomial...
Questions

Mathematics, 28.08.2019 06:10

English, 28.08.2019 06:10

Mathematics, 28.08.2019 06:10


Mathematics, 28.08.2019 06:10

History, 28.08.2019 06:10






Mathematics, 28.08.2019 06:10

History, 28.08.2019 06:10





Mathematics, 28.08.2019 06:10