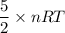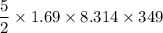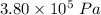A tank contains 120.0 g chlorine gas (Cl2), which is at temperature 76.0°C and absolute pressure 5.70 ✕ 105 Pa. The temperature of the air outside the tank is 19.0°C. The molar mass of Cl2 is 70.9 g/mol. (a) What is the volume of the tank (in m3)? m3 (b) What is the internal energy of the gas (in J)? J (c) What is the work done by the gas (in J) if the temperature and pressure inside the tank drop to 31.0°C and 3.80 ✕ 105 Pa, respectively, due to a leak? (Assume that the air outside the tank can be treated as a vacuum.)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1

Chemistry, 23.06.2019 05:30
Astudent made the lewis dot diagram of a compound as shown. mg is written with two dots shown on its top. an o is written on each side of mg. each o has six dots around it. an arrow is shown from one dot on mg toward the vacant space around the o on the right. another arrow is shown from the other dot on mg toward the vacant space around the o on the left. the title of the art is students lewis dot model. what is the error in the lewis dot diagram? an o atom should transfer all its six electrons to mg because the formula is mgo. both electrons of mg should be transferred to one o atom because the formula is mgo. the electrons should be transferred from each o atom to mg because mg has fewer electrons. the number of dots around mg should be four because it has to transfer two electrons to each o.
Answers: 2

Chemistry, 23.06.2019 09:30
The allotropes of carbon include a variety of structures that include three-dimensional tetrahedral lattices, planes of hexagonal rings, cylindrical tubes of hexagonal rings, and spheres of five- and six-membered rings. similar shapes of network covalent atomic solids are possible with carbon nitride, boron, and pure silicon (e.g., silicene is a graphene-like allotrope of pure silicon). in contrast, silicates exist as either highly ordered or amorphous (more random) three-dimensional lattices. what could explain why there are there no naturally occurring sheets, stacked sheets, cylindrical tubes, or spheres of network covalent atomic solids composed of silicon and oxygen (sio2)? would pure silicate structures make good lubricants or good electrical conductors?
Answers: 3
You know the right answer?
A tank contains 120.0 g chlorine gas (Cl2), which is at temperature 76.0°C and absolute pressure 5.7...
Questions


English, 19.06.2021 17:30


Mathematics, 19.06.2021 17:40


Mathematics, 19.06.2021 17:40

English, 19.06.2021 17:40

Mathematics, 19.06.2021 17:40

Mathematics, 19.06.2021 17:40

Mathematics, 19.06.2021 17:40

Physics, 19.06.2021 17:40

Physics, 19.06.2021 17:40



Mathematics, 19.06.2021 17:40


English, 19.06.2021 17:40


Business, 19.06.2021 17:40

Mathematics, 19.06.2021 17:40

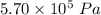
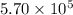
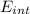 =
=