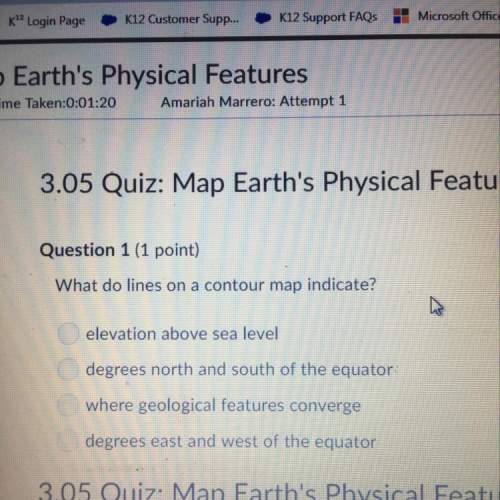
How many σ and π bonds are in this molecule? A chain of five carbon atoms. There is a double bond between the first and second carbon atoms and a triple bond between the fourth the fifth carbon atoms. There are single bonds between the remaining carbon atoms. There are two hydrogen atoms bonded to the first carbon atom through single bonds, and a single hydrogen atom bonded to both the second and fifth carbon atoms through single bonds. There is an oxygen atom bonded to the third carbon atom through a double bond.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:40
Which of the following might a chemist choose to study? a. glacier movement in alaska b. better ways to recycle plastics c. the effects of hurricanes on turtle populations d. the vibrations in bridges caused by big trucks
Answers: 2

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1
You know the right answer?
How many σ and π bonds are in this molecule? A chain of five carbon atoms. There is a double bond be...
Questions




Mathematics, 08.12.2019 17:31

Mathematics, 08.12.2019 17:31

English, 08.12.2019 17:31


Social Studies, 08.12.2019 17:31

Mathematics, 08.12.2019 17:31


English, 08.12.2019 17:31

History, 08.12.2019 17:31



Chemistry, 08.12.2019 17:31



Biology, 08.12.2019 17:31

History, 08.12.2019 17:31

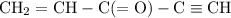 .
.