
Chemistry, 08.10.2020 08:01 michaelgold1
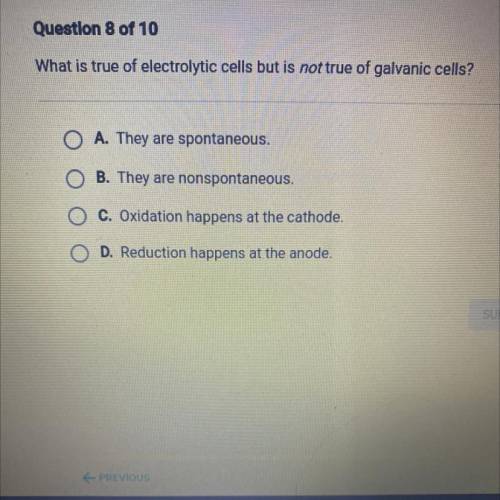
What is true of electrolytic cells but is not true of galvanic cells?
O A. They are spontaneous.
O B. They are nonspontaneous.
O C. Oxidation happens at the cathode.
O D. Reduction happens at the anode.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
What is true of electrolytic cells but is not true of galvanic cells?
O A. They are spontaneous.
Questions


Mathematics, 08.02.2021 05:40


English, 08.02.2021 05:40

Mathematics, 08.02.2021 05:40

Social Studies, 08.02.2021 05:40

Mathematics, 08.02.2021 05:40

Biology, 08.02.2021 05:40


Biology, 08.02.2021 05:40

Mathematics, 08.02.2021 05:40

Mathematics, 08.02.2021 05:40

History, 08.02.2021 05:40


Mathematics, 08.02.2021 05:40

Social Studies, 08.02.2021 05:40

Mathematics, 08.02.2021 05:40

Mathematics, 08.02.2021 05:40


Mathematics, 08.02.2021 05:50



