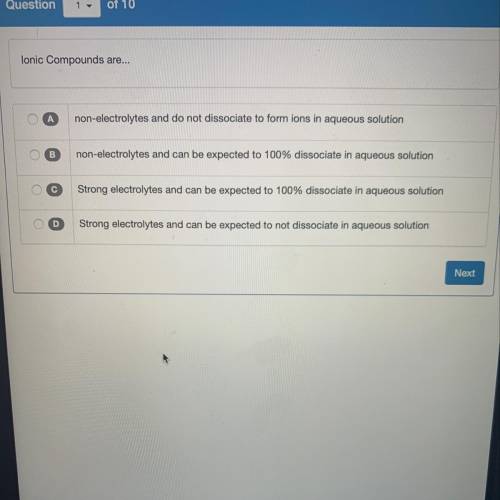
Lonic Compounds are...
A
non-electrolytes and do not dissociate to form ions in aqueous solut...

Chemistry, 10.10.2020 16:01 sbelgirl2000
Lonic Compounds are...
A
non-electrolytes and do not dissociate to form ions in aqueous solution
B
non-electrolytes and can be expected to 100% dissociate in aqueous solution
с
Strong electrolytes and can be expected to 100% dissociate in aqueous solution
D
Strong electrolytes and can be expected to not dissociate in aqueous solution
Next

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1


Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
You know the right answer?
Questions


Arts, 13.01.2021 17:50


Health, 13.01.2021 17:50



Health, 13.01.2021 17:50

English, 13.01.2021 17:50

Mathematics, 13.01.2021 17:50

Mathematics, 13.01.2021 17:50


Mathematics, 13.01.2021 17:50


Mathematics, 13.01.2021 17:50





Computers and Technology, 13.01.2021 17:50

English, 13.01.2021 17:50



