check all that apply.

Why are most organic compounds nonconducting and insoluble in water?
check all that apply.
most organic compounds don't dissolve in water because they are polar.
most organic compounds don't dissolve in water because they are nonpolar.
they don't conduct electricity because they are ionic, not covalent.
they don't conduct electricity because they are covalent, not ionic.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2
You know the right answer?
Why are most organic compounds nonconducting and insoluble in water?
check all that apply.
check all that apply.
Questions


Chemistry, 04.12.2019 22:31

English, 04.12.2019 22:31


Mathematics, 04.12.2019 22:31


Mathematics, 04.12.2019 22:31







History, 04.12.2019 22:31






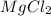 etc that are also polar in nature.
etc that are also polar in nature.

