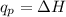Identify each enthalpy change by name and classify each change as exothermic or endothermic.
1. 1 mol nacl (s) à 1 mol nacl(l)
2. c4h10 o(l) à c4h10o (g)
3. h2o(g) à h2o (l)
4. 1 mol nacl(s) + 3.88 kj/molà 1 mol nacl(aq)
a molar heat of solution; endothermic
b molar heat of vaporization; endothermic
c molar heat of fusion; endothermic
d molar heat of condensation; exothermic

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
Identify each enthalpy change by name and classify each change as exothermic or endothermic.
Questions

History, 10.01.2021 01:10

Mathematics, 10.01.2021 01:10

Mathematics, 10.01.2021 01:10


Chemistry, 10.01.2021 01:10




Mathematics, 10.01.2021 01:10

Mathematics, 10.01.2021 01:10


Mathematics, 10.01.2021 01:10



Chemistry, 10.01.2021 01:10

English, 10.01.2021 01:10




Computers and Technology, 10.01.2021 01:10