
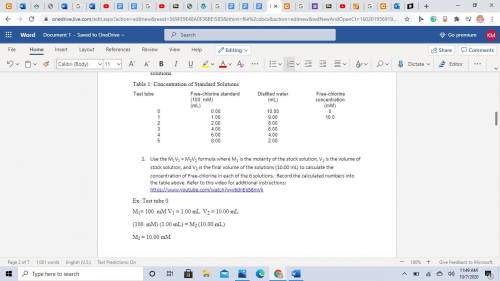
A 100mM/L solution of Free-chlorine stock solution was used to make up 6 standard solutions (known concentration). See the table below with gives the test tube number, the amount of stock solution used and the amount of distilled water used to make up these solutions.
Table 1: Concentration of Standard Solutions
Test tube
Free-chlorine standard (100. mM)
(mL)
Distilled water
(mL)
Free-chlorine concentration
(mM)
0
0.00
10.00
0
1
1.00
9.00
10.0
2
2.00
8.00
3
4.00
6.00
4
6.00
4.00
5
8.00
2.00
Use the M1V1 = M2V2 formula where M1 is the molarity of the stock solution, V1 is the volume of stock solution, and V2 is the final volume of the solutions (10.00 mL) to calculate the concentration of Free-chlorine in each of the 6 solutions. Record the calculated numbers into the table above. Ex: Test tube 0
M1= 100. mM V1 = 1.00 mL V2 = 10.00 mL
(100. mM) (1.00 mL) = M2 (10.00 mL)
M2 = 10.00 mM
(there's also a screenshot of the problem if that's easier)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
A 100mM/L solution of Free-chlorine stock solution was used to make up 6 standard solutions (known c...
Questions

Computers and Technology, 02.08.2019 03:30

History, 02.08.2019 03:30



Spanish, 02.08.2019 03:30


Mathematics, 02.08.2019 03:30

Advanced Placement (AP), 02.08.2019 03:30


History, 02.08.2019 03:30

Spanish, 02.08.2019 03:30

History, 02.08.2019 03:30



Physics, 02.08.2019 03:30


English, 02.08.2019 03:30

Mathematics, 02.08.2019 03:30

Mathematics, 02.08.2019 03:30

Mathematics, 02.08.2019 03:30



