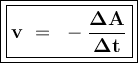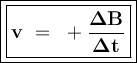Chemistry, 12.10.2020 22:01 aubreystechschu11331
A student measures the time it takes for two reactions to be completed. Reaction A is completedd in 12 seconds, and reaction B is completed in 2q seconds. What can you conclude about the rates of the reactions?
A. The rate of reaction A is lower
B. The rates of reation A and B are equal
C. The rate of reaction B is lower

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1


Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
You know the right answer?
A student measures the time it takes for two reactions to be completed. Reaction A is completedd in...
Questions


Mathematics, 26.02.2021 18:10

Mathematics, 26.02.2021 18:10



Mathematics, 26.02.2021 18:10




Arts, 26.02.2021 18:10

Mathematics, 26.02.2021 18:10



History, 26.02.2021 18:10

History, 26.02.2021 18:10


English, 26.02.2021 18:10


English, 26.02.2021 18:10

Mathematics, 26.02.2021 18:10